Applications of SANS in nanoscience: Difference between revisions
ucph>Tommy No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 2: | Line 2: | ||
==Polymers== | ==Polymers== | ||
<figure id="fig:JJKK1">[[File: | <figure id="fig:JJKK1">[[File:Fig1a.png|thumb|300px|<caption>Examples of polymers: PE and PVC are synthetic polymers produced industrially, the polypeptide is an example of a biological polymer. Figures from <ref name="sneppen">Kim Sneppen and Giovanni Zocchi. ''Physics in Molecular Biology''. Cambridge University Press, 2005.</ref><!--\cite{sneppen}-->.</caption>]]</figure> | ||
A very important class of molecules are polymers. As the name suggests, a polymer is built by assembling many chemical building blocks: ''monomers''. Typically, this would be into single long chains, although a number of branched architectures also exist. The chains are formed by chemical bonds between the monomers. Conceptually you can think of polymers as beads on a string. Almost everything surrounding us in our daily life is made of polymers, for example plastics, rubber (typically built from one or two types of monomers), and biological molecules like DNA and proteins/peptides (built from several to many types of monomers). <xr id="fig:JJKK1">Figure %i</xr><!--Fig.~\ref{fig:JJKK1}--> shows examples of two simple plastic types (polyethylene and PVC), as well as an example of a peptide/protein-like structure. | A very important class of molecules are polymers. As the name suggests, a polymer is built by assembling many chemical building blocks: ''monomers''. Typically, this would be into single long chains, although a number of branched architectures also exist. The chains are formed by chemical bonds between the monomers. Conceptually you can think of polymers as beads on a string. Almost everything surrounding us in our daily life is made of polymers, for example plastics, rubber (typically built from one or two types of monomers), and biological molecules like DNA and proteins/peptides (built from several to many types of monomers). <xr id="fig:JJKK1">Figure %i</xr><!--Fig.~\ref{fig:JJKK1}--> shows examples of two simple plastic types (polyethylene and PVC), as well as an example of a peptide/protein-like structure. | ||
| Line 20: | Line 20: | ||
==Lipids and surfactants== | ==Lipids and surfactants== | ||
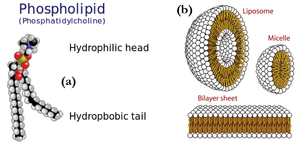
<figure id="fig:JJKK2">[[File: | <figure id="fig:JJKK2">[[File:Fig2aandfig2b.png|thumb|300px|<caption>(a) Example of amphiphile: a phospholipid. (b) Aggregates formed by self-assembled lipids. A vesicle or liposome, a micelle and a bilayer sheet. Figures from Wikipedia.</caption>]]</figure> | ||
Other polymer-related classes of materials comprise lipids and surfactants, which play a significant role in biology and nano-science. These consist of amphiphilic molecules, ''i.e.'' they are made from components that are for example hydrophobic and hydrophilic (water hating and loving, respectively). Examples of such molecules are shown in <xr id="fig:JJKK2">Figure %i</xr>(a)<!--Fig.~\ref{fig:JJKK2}(a)-->. | Other polymer-related classes of materials comprise lipids and surfactants, which play a significant role in biology and nano-science. These consist of amphiphilic molecules, ''i.e.'' they are made from components that are for example hydrophobic and hydrophilic (water hating and loving, respectively). Examples of such molecules are shown in <xr id="fig:JJKK2">Figure %i</xr>(a)<!--Fig.~\ref{fig:JJKK2}(a)-->. | ||
Latest revision as of 16:38, 20 April 2020
We here start with a few introductory examples to the types of science, where small-angle neutron scattering is useful.
Polymers
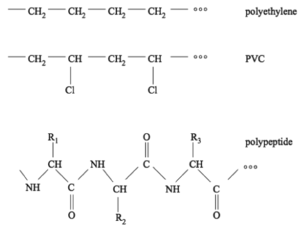
A very important class of molecules are polymers. As the name suggests, a polymer is built by assembling many chemical building blocks: monomers. Typically, this would be into single long chains, although a number of branched architectures also exist. The chains are formed by chemical bonds between the monomers. Conceptually you can think of polymers as beads on a string. Almost everything surrounding us in our daily life is made of polymers, for example plastics, rubber (typically built from one or two types of monomers), and biological molecules like DNA and proteins/peptides (built from several to many types of monomers). Figure xx--CrossReference--fig:JJKK1--xx shows examples of two simple plastic types (polyethylene and PVC), as well as an example of a peptide/protein-like structure.
Some of the most important characteristics of polymers are structural signatures like their size, shape and flexibility, often under different circumstances like varying temperature. For example, polyethylene is very flexible, while DNA is very stiff. The size of the polymer relates to its length, also called the degree of polymerization, i.e. how many monomers, \(N\), it is built from. A typical size measure in polymer science is the radius of gyration which is generally defined as the root mean square distance \(R_g\) of an object's parts from its center of mass
\begin{equation}\label{eq_rg} R_g^2 = \frac{1}{N}\sum_{n=1}^N \langle (\bar{R}_n-\bar{R}_G)^2 \rangle, \end{equation}
where \(R_n\) and \(R_G\) are the position vectors for the object parts and its centre of mass respectively. For a so-called ideal chain polymer this is given by
\begin{equation} R_g^2 = \frac{1}{6}Nb^2, \end{equation}
where \(b\) is the polymer segment (bond) length. For non-ideal (real) chains the size scaling does not obey \(R_g \propto N^{1/2}\) but depends on external parameters, for example whether it is surrounded by a solvent. The structural properties of polymers are typically investigated by scattering methods.
Lipids and surfactants
Other polymer-related classes of materials comprise lipids and surfactants, which play a significant role in biology and nano-science. These consist of amphiphilic molecules, i.e. they are made from components that are for example hydrophobic and hydrophilic (water hating and loving, respectively). Examples of such molecules are shown in Figure xx--CrossReference--fig:JJKK2--xx(a).
Lipids are extremely important in biology as they are the building blocks of cells and membranes. Amphiphilic molecules self-assemble in solution to form aggregated structures. The shape and size of these structures can be explored in detail using scattering methods. Figure xx--CrossReference--fig:JJKK2--xx(b) show examples of some of the simplest lipid aggregates, namely micelles and vesicles.
- ↑ Kim Sneppen and Giovanni Zocchi. Physics in Molecular Biology. Cambridge University Press, 2005.