Problem: Use of International Tables for Crystallography: Difference between revisions
ucph>Tommy (Created page with "<figtable id="tab:bentetabel1"> File:bentetabel1.png | thumb | 200px | <caption>Table to be filled in during the problem [[Exercises in Diffraction from crystals#Problem: Us...") |
m (1 revision imported) |
(No difference)
| |
Latest revision as of 22:15, 18 February 2020
This exercise is intended to illustrate how the symmetry information of a particular crystal lattice and space group listed in "International Tables for Crystallography" can be used to visualise and understand the crystal structures of very different chemical compounds.
Question 1
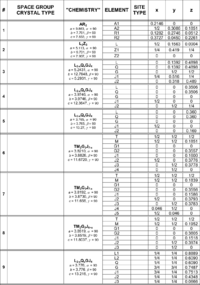
Table xx--CrossReference--tab:bentetabel1--xx contains six filled and two unfilled columns. The first column is just a running number used to identify the different compounds. The third column quotes a fictitious chemical formula for the compound and the lattice parameters. The fourth and last three columns list the individual elements with the corresponding crystal lattice coordinates \(x\), \(y\), \(z\). Simple letter synonyms are used for the elements. A selection of information sheets from "International Tables for Crystallography" can be found in the course material. Use the information for space groups #47, #64, #123, #129, #139, #194 and #225 in conjunction with the section How to use the information in IT on the Diffraction from crystals page to fill in the missing information in column two (space group and lattice type) and five (type of site). When this is done, answer the following questions.
Question 2
Must all atoms of the same element be on the same site? Why can chemically very different compounds be described in by the same space group?
Question 3
In some of the compounds, the coordinates are identical for different elements. What does that mean? Many of the compounds have different elements in the same site positions. How is that possible?
Question 4
The space groups #6, 7 and 8 (Table xx--CrossReference--tab:bentetabel1--xx) contain the same elements (\(x > 1\)). What is the difference between these structures? How many atom sites of the element \(\mathbf J\) does each structure contain? Are these numbers consistent with the chemistry? What does this conclusion imply?
Question 5
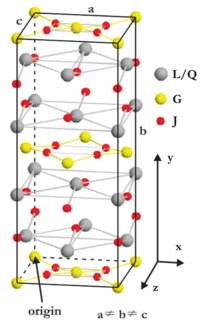
Figure xx--CrossReference--fig:useofITcrystal--xx shows the unit cell crystal structure for one of the structures listed in Table xx--CrossReference--tab:bentetabel1--xx. Look at the figure and compare the atom positions with the site positions listed in the filled in Table xx--CrossReference--tab:bentetabel1--xx. Determine the number (first column of Table xx--CrossReference--tab:bentetabel1--xx) and space group for this structure. Argue for the result.
Question 6
From a symmetry point of view, it seems that the structure from the previous questioncontains more than one type (site) of \(\mathbf J\)-atoms. How many types are there? Is there a simple way to describe them in relation to the crystal lattice[1]? Is this consistent with the filled Table xx--CrossReference--tab:bentetabel1--xx? Does the total number of each atom type agree with number of site positions in Table xx--CrossReference--tab:bentetabel1--xx?
Question 7
What are the occupancies of the \(\mathbf L\)-, \(\mathbf Q\)-, \(\mathbf G\)- and \(\mathbf J\)-atom sites if \(x = 0.14\) and the information is to be used as input in a FullProf refinement (see the section on Occupancy on the Diffraction from crystals page)? Answer the same question if the information will be used as input in Crystallographica.
- ↑ E.g. central, face centre, body centre, in planes parallel to a lattice plane or on lines parallel to a direction.